Tirzepatide: Semaglutide 2.0 For Fat Loss And Insulin-Controlled Living

It was only 10 months ago when I introduced Semaglutide to the world as the best agent for blunting your appetite (although I later argued Tesofensine was even better).
But in the middle of 2022, Christopher Mercer of Limitless Life Nootropics told me about a newcomer drug to the weight loss industry called Tirzepatide.
His unnamed friend, an owner of a weight loss clinic, stated all her patients were switching from Semaglutide to Tirzepatide for three reasons: More weight loss in the same period of time time, heavier suppression of appetite, and fewer side effects.
I thought Tirzepatide was going to be another “fad” drug that comes as quickly as it goes…
… until I hit up hormone specialist Dr. Rudolph Eberwein of the Medical Health Institute (MHI) clinic and his partners Michael and Carlos Bertonatti the owners of MHI.
I started using Tirzepatide on October 9th, and nearly 3 weeks later all I have to say is HOLY F*CKING SH*T!
This is undoubtedly Semaglutide 2.0 with enhanced upgrades and bug fixes.
Not only is the appetite suppression with this peptide off-the-charts, but I’m 100% convinced there is some extra thermogenesis going on.
Thanks to my short-lived results, combined with 17 years worth of clinical experience from Dr. Eberwein, we’re working together to give you the scoop on what is unquestionably a true weight-loss breakthrough.
What is Tirzepatide?

Tirzepatide is a synthetic peptide that is 39 amino acids in length:
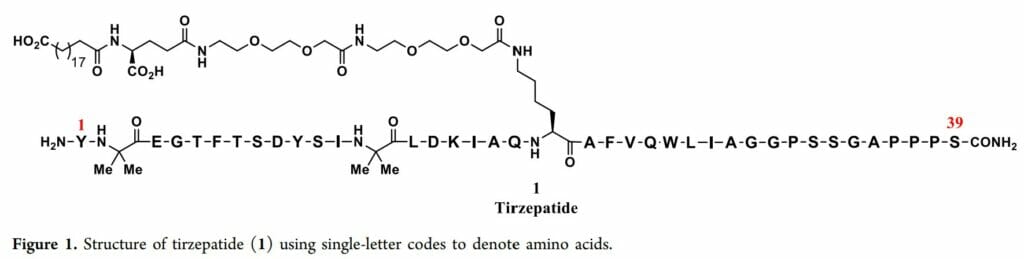
(Source)
Just for the science geeks, here’s the entire structure of Tirzepatide without any symbols representing amino acids to save space:
(Source)
But before I can tell you exactly why it’s the revolutionary “2nd generation” weight loss gamechanger, we need to take a brief trip back into the history of developing pharmaceuticals for losing weight.
When Dr. Eberwein first started his weight loss clinic in 2005 (~17 years ago), the available drugs were all focused on the suppression of appetite.
If you can get people to “just stop” being hungry, they won’t eat as much, and the pounds will just drop off.
The majority of drugs designed to target this problem were stimulants, an approach that had solid evidence to back up its efficacy:
“Stimulants activate reward mechanisms by interacting with amine transporter systems, decreasing the synaptic reuptake of dopamine and noradrenaline, and to a lesser extent serotonin so that neurotransmission is enhanced. This results in mundane tasks appearing more rewarding.
Stimulants are also associated with appetite suppression, which correlates with the increased activity of the mesolimbic reward pathway and elevated extracellular dopamine the nucleus accumbens. Therefore, stimulants address the reward deficit, which may rectify the compulsion to eat excessively and could also explain their positive effect on mood“
Drugs in this class include well-known names like Phentermine (and the infamous “FenPhen” combination).
Unfortunately, they all suffered from the same types of problems: Causing high blood pressure, inducing heart problems, great potential for addictive use and abuse, unsatisfactory weight loss, and weight regain upon cessation of use.
You also have drugs like Orlistat for preventing fat absorption in the gut and antidepressants such as Wellbutrin that can be repurposed as a weight loss solution…
…but other than those few examples, the weight loss industry has been starving (no pun intended) for a real long-lasting change.
This is when I introduce glucagon-like peptide 1 (GLP-1), which has turned out to be the missing key in the seemingly unsolvable equation of weight loss:
“The development of the ‘incretin concept’ which hypothesised that hormones from the gut contributed to the insulin secretion in response to meals, led to the identification of glucagon-like peptide 1 (GLP-1) as an important ‘incretin’ hormone.
GLP-1 not only increases insulin secretion but increases β-cell proliferation and survival, suppresses glucagon secretion, delays gastric emptying and suppresses appetite, all of these actions contributing to a potential anti-diabetic effect.
However, GLP-1 has a very short half due to its rapid breakdown by dipeptidyl peptidase IV and ectopeptidases. “
And here is some further background on incretin hormones, which explains why the discovery of GLP-1 was such a big deal for treating diabetes:
“Incretin hormones are gut peptides that are secreted after nutrient intake and stimulate insulin secretion together with [decreased blood sugar]. GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) are the known incretin hormones from the upper (GIP, K cells) and lower (GLP-1, L cells) gut.
Together, they are responsible for the incretin effect: a two- to three-fold higher insulin secretory response to oral as compared to intravenous glucose administration.
In subjects with type 2 diabetes, this incretin effect is diminished or no longer present. This is the consequence of a substantially reduced effectiveness of GIP on the diabetic endocrine pancreas, and of the negligible physiological role of GLP-1 in mediating the incretin effect even in healthy subjects.
However, the insulinotropic and glucagonostatic effects of GLP-1 are preserved in subjects with type 2 diabetes to the degree that pharmacological stimulation of GLP-1 receptors significantly reduces plasma glucose and improves glycaemic control.”
(We’ll get to GIP later, but for now let’s focus on GLP-1)
To put all of that in simple English… we can stimulate the receptors that GLP-1 attaches to and get all of the downstream effects (decreased appetite, blood sugar regulation, etc.).
And even though GLP-1 secretion may not be related to the onset of Type 2 Diabetes, its secretion from the gut is negatively affected in obesity.
This led to the creation of the first FDA-approved GLP-1 receptor agonist known as “Exenatide” (a.k.a. Byetta):
“The first GLP-1 receptor agonist to be approved for the treatment of type 2 diabetes was exenatide [in 2005], which was synthetic exendin-4, a peptide from the saliva of a lizard from Arizona, Heloderma suspectum, which was purified without any intention to be in search of a diabetes medication.
The similarity of the peptide structure to GLP-1 was noticed, and it was found to stimulate GLP-1 receptors with an affinity like (or even slightly better than) GLP-1. Exenatide happened to be DPP-4-resistant and eliminated more slowly, with a half-life of approximately 2–3 h”
(I recommend reading the paper above to see a brief history of all the GLP-1 receptor agonists developed and approved for the treatment of Type 2 Diabetes)
It was a breakthrough medication for diabetics and a new way to treat patients with bad blood markers, and its weight loss results were okay, but the first iteration isn’t always the best iteration.
Several GLP-1 receptor agonists were developed with the intention of far less frequent dosing (i.e. once-a-week) for equal if not superior results, and eventually we ended up with Semaglutide.
I don’t want to get too deep into pharmacokinetics, but the long story short is that Semaglutide (best known as Ozempic or Wegovy) accelerated weight loss in a way its predecessors could not.
And that’s how in June 2021, it became the first FDA-approved weight management drug since 2014.
What Makes Tirzepatide Different From Semaglutide?
I touched upon Tirzepatide briefly two weeks ago as the superior version of Semaglutide, but I want to expand on exactly why it arguably pioneered the next evolutionary stage of weight loss drugs.
It has to do with the glucose-dependent insulinotropic peptide (GIP) I mentioned earlier, as originally it was an unviable drug target due to its then-understood role in the incretin effect:
“GIP is the main incretin hormone in healthy people, causative of most the incretin effects but the insulin response after GIP secretion in T2DM [type 2 diabetes meillus] is reduced.
It has been reported that there is no reduction in its secretion in patients with T2DM but a nearly total loss of insulinotropic effect is observed, even at supraphysiological concentrations, implying the existence of GIP resistance“
This changed when emerging research revealed GIP resistance was NOT irreversible:
“…it has been reported that resistance to GIP can be reversed and its effectiveness restored by improving glycemic control.
A potential additional advantage of GIP is the protection against hypoglycemia since GIP infusion is accompanied by a decreased need for further glucose administration to maintain a satisfactory glycemic level during an insulin-induced hypoglycemic clamp.
Moreover, it improves triglyceride clearance and the sensitivity of adipose tissue to insulin, which may prevent ectopic fat deposition“
With this knowledge in mind, why not create a compound that is a powerful agonist for both the GIP and GLP-1 receptors?
Pharmaceutical company Eli Lilly pursued this novel idea, eventually creating the “twincretin” known as Tirzepatide (brand name Mounjaro).
But what’s interesting is how the peptide is “imbalanced” with respect to its affinity for the two receptors:
“Tirzepatide was discovered by engineering GLP-1 activity into the GIP sequence. It is an imbalanced dual agonist in favor of GIPR over GLP-1R activity as the molecule shows equal affinity for the GIPR compared with native GIP but binds the GLP-1R with approximately 5-fold weaker affinity than native GLP-1.
The imbalanced nature of tirzepatide may be critical to maximizing the efficacy of a dual agonist because dose escalation for GLP-1R activation can be limited by gastrointestinal effects such as nausea and vomiting, while GIPR engagement is not known to be associated with similar events.
As a consequence, a pharmacological profile favoring potency at the GIPR offers an opportunity to fully engage this pathway while minimizing GLP-1–related tolerability issues.”
With promising data for this approach over targeting a single receptor in animals, the only thing left to do was put Tirzepatide head-on against the recently-approved Semaglutide in obese/overweight patients with or without diabetes.
Tirzepatide’s Mechanism of Action

By now, it’s blatantly clear that Tirzepatide works by selectively binding to the GIP and GLP-1 receptors.
What’s unclear is where the GLP-1 and GIP receptors are unique with respect to their downstream effects.
(Source)
First, it appears that while GLP-1 activity is systemic in nature, GIP activity is primarily focused around the pancreas.
Second, it seems like GIP may have a larger effect on body fat tissue in addition to its regulation of glucagon and insulin:
(Source)
However, given that both receptors are prominent in numerous parts of the body, it’s easy to see why they both appear to be working together synergistically:
“[GLP-1 is] synthesised in the L cells of the intestine. Once released into the circulation, GLP-1 binds to a specific GLP-1 receptor, which is expressed in the pancreas, gastrointestinal tract, kidney, heart and brain. Endogenous GLP-1 has a short half-life of 1–2 min and is metabolised by the enzyme DPP4.
In additional to the well-known insulinotropic property in response to nutrients in the gut, GLP-1 has a glucagonostatic effect, inhibiting glucagon secretion in the hyperglycaemic and normoglycaemic state but not during hypoglycaemia”
GIP is only slightly different from GLP-1, but different enough that it has its own functions and contributes towards bodily homeostasis in its own way:
“[GIP is] produced by the K cells of the intestine, mainly located in the duodenum and proximal jejunum. GIP is released in response to oral nutrients, especially carbohydrates and lipids. GIP, like GLP-1, has a short half-life (4–7 min) and is inactivated by DPP4, shortly after release. GIP receptors are present in various tissues such as the pancreas, adipose tissue, gastric mucosa, heart, adrenal cortex, bone and brain
…One difference between GIP and GLP-1 is the effect on glucagon secretion. Unlike GLP-1, GIP has dual functions: a glucagonotropic property in the normoglycaemic and hypoglycaemic state, and glucagonostatic in the hyperglycaemic state”
We can’t be 100% sure how both GIP and GLP-1 aid with weight loss — all we know is that Tirzepatide does something to our metabolism in addition to decreasing our caloric intake via the increase in satiety.
And thanks to its half-life of just 5 days, all you have to do is dose the peptide once a week to reap its full health benefits.
Top 2 Health Benefits of Tirzepatide

Tirzepatide appears to be the perfect weight loss medication for people who are overweight and obese, with or without diabetes.
Additionally, we may have something in place that can also improve our most critical biomarkers, which would make it an antidiabetic therapeutic.
The only question is how well Tirzepatide fares against placebo, existing diabetes treatments, and existing weight loss drugs (especially the 1st generation GLP-1 receptor agonists).
Fortunately, we more-or-less have all the answers in the SURPASS and SURMOUNT clinical trials — some have been fully completed and other are ongoing.
Let’s see if GIP is contributing to weight loss more than preliminary research anticipates…
Tirzepatide Provides Superior Weight Loss
SURPASS-1 was a Phase III double-blind randomized trial where 478 adults with Type 2 Diabetes either took a placebo, 5 mg of Tirzepatide weekly, 10 mg of Tirzepatide weekly, or 15 mg of Tirzepatide weekly over 40 weeks.
But get this… not only did the patients’ bodyweight have to stay stable (fluctuations below 5%) in the 90 days before the study, they also agreed not to follow any exercise or diet program that would reduce bodyweight.
And the results obtained with Tirzepatide were very surprising to say the least:
- Clinically significant reductions in glycated haemoglobin (HbA1c) regardless of dose
- “92% of participants on Tirzepatide achieved an A1C under 7%… and 52% achieved an A1C under 5.7%—the level for people without diabetes” (Source)
- Improvements in fasting serum glucose as early as four weeks
- 7.0-9.5 kg bodyweight loss compared to 0.7 kg from placebo, and weight loss was not dose-dependent but did not plateau after 40 weeks had gone by
- 67-78% of patients on Tirzepatide achieved final bodyweight reductions of 5% or higher
- 31-47% of patients on Tirzepatide achieved final bodyweight reductions of 10% or higher
- 13-27% of patients on Tirzepatide achieved final bodyweight reductions of 15% or higher
However… the Phase III SURPASS-2 trial was the real test, because Tirzepatide was compared head-on with Semaglutide to determine if it was the true “game changer” for the weight loss industry.
Would Semaglutide being the better GLP-1 receptor agonist end up putting Tirzepatide down a peg? Or was it time for a brand new contender to shine?
Over 40 weeks, 1879 adults with Type 2 Diabetes who had failed to control the disease with Metformin (another story for another time) were randomly assigned to 1 mg Semaglutide weekly, 5 mg Tirzepatide weekly, 10 mg Tirzepatide weekly, or 15 mg Tirzepatide weekly.
Just like SURPASS-1, patients needed to have stable bodyweight (±5%) for the 3 months leading up to the trial start date and no diet/exercise modifications were made.
Thanks to the authors of this study, we have a neat infographic showcasing the results:
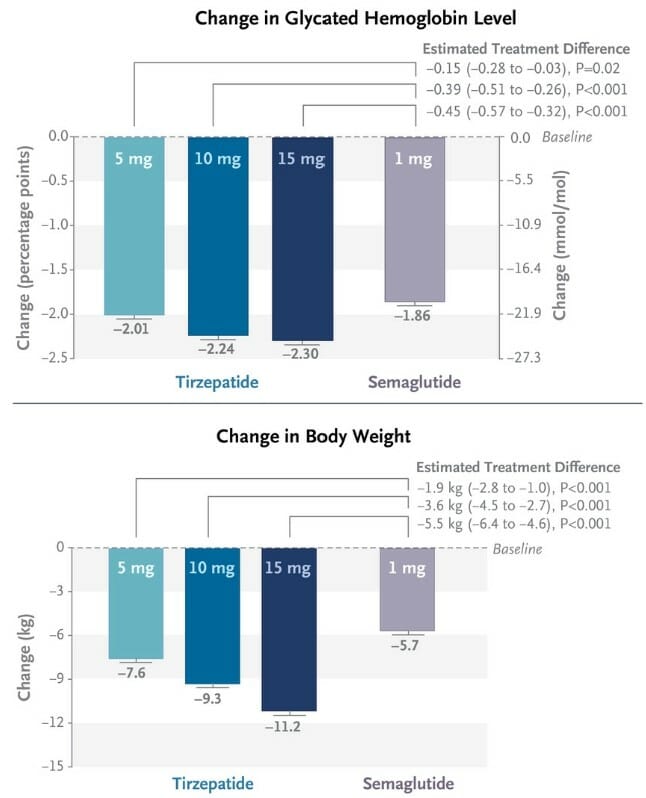
LOOK AT THAT – 24 entire pounds lost over the 40 weeks on the highest dose of Tirzepatide, compared to the ~13 pounds lost on Semaglutide!
However, there are two important things to note about the study:
- DOSE ESCALATION – All patients in the Tirzepatide groups were initially dosed at 2.5 mg weekly, and doses increased by 2.5 mg every 4 weeks until their randomly assigned dose was reached (and maintained for the trial). Likewise, all patients in the Semaglutide group were initially dosed at 0.25 mg weekly, and dose was doubled every 4 weeks until 1 mg was reached (and maintained for the trial)
- SEMAGLUTIDE DOSING – The highest possible dose of Semaglutide was not compared with the highest possible dose of Tirzepatide, so we won’t know how much of a difference a 2.0-2.4 mg dose of Semaglutide would have made.
Last but not least, the Phase III SURMOUNT-1 clinical trial looked at 2539 obese adults who did not have diabetes.
They received either a placebo, 5 mg Tirzepatide weekly, 10 mg Tirzepatide weekly, or 15 mg Tirzepatide weekly for 72 weeks straight (including 20 weeks for dose escalation as described in SURPASS-2).
Patients were not allowed to have had a bodyweight change of more than 5 kg or taken any weight loss promoting medication 3 months before the trial started.
The weight loss results continued to amaze the weight loss community:
“The mean percentage change in weight at week 72 was −15.0% with 5-mg weekly doses of tirzepatide, −19.5% with 10-mg doses, and −20.9% with 15-mg doses and −3.1% with placebo.
The percentage of participants who had weight reduction of 5% or more was 85%, 89%, and 91% with 5 mg, 10 mg, and 15 mg of tirzepatide, respectively, and 35% with placebo;
50% and 57% of participants in the10-mg and 15-mg groups had a reduction in body weight of 20% or more, as compared with 3% in the placebo group”
All of the weight loss was significant in how much was lost, AND it was fully sustained.
Folks, you can’t even begin to understand how much of a miracle this is for a weight loss physician.
These are the types of numbers that can lead to someone seriously considering Tirzepatide over a gastric bypass.
Nothing else in the WORLD can control appetite so well to the point where the pleasure from eating food is taken away, which eliminates snacking and naturally lowers how much food you eat.
Combine this with nutrition education and behavior modification, and the results will blow the minds of both obese and non-obese people.
It’s no wonder that in May 2022 Tirzapetide got FDA-approved for treating Type 2 Diabetes, and a Fast Track Designation in October 2022 for treating non-diabetic obese adults!
Tirzepatide Improves Your Blood Work
While the remaining SURPASS trials still examined weight loss for diabetics in comparison to other pre-existing treatments, I want to focus on just how consistently glycemic control improves and insulin dependency lessens.
So without copy-pasting the entire trials word-for-word, I’m just going to share the key highlights…
SURPASS-3 (full trial can be found here):
- 1437 Type 2 Diabetes patients were recruited who took Metformin with/without an SGLT2 inhibitor 90 days before the study started
- Patients received a subcutaneous injection of Tirzepatide weekly (5, 10, or 15 mg) or an injection of titrated insulin degludec daily for 52 weeks
- Tirzepatide users were far less likely to experience hypoglycemia compared to degludec users (1.1-2.2% vs. 7.3%)
- Tirzepatide users reduced HbA1c levels by 1.93, 2.20, and 2.37 percentage points with the 5, 10, and 15 mg/week doses (compared to 1.34 for placebo)
SURPASS-4 (full trial can be found here):
- 1995 Type 2 Diabetes patients of ~10 years with established high risk of cardiovascular events
- Patients received a subcutaneous injection of Tirzepatide weekly (5, 10, or 15 mg) or an injection of titrated insulin glargine
- “Blood pressure decreased with tirzepatide but increased with glargine, and tirzepatide treatment also reduced levels of atherogenic lipids, compared with ‘marginal changes’ in people taking glargine.”
- “74–88% of people taking tirzepatide achieved HbA1c below 7.0% (53 mmol/mol) without weight gain or clinically significant or severe hypoglycemia, compared with just 13% of those given glargine”
SURPASS-5 (full trial can be found here)
- 475 Type 2 Diabetes patients who are already taking insulin glargine with/without Metformin
- Patients received a subcutaneous injection of Tirzepatide weekly (5, 10, or 15 mg) or pacebo
- “People randomly assigned to take tirzepatide… achieved HbA1c reductions averaging 2.23, 2.59, and 2.59 percentage points with the 5, 10, and 15 mg/week doses”
- “The vast majority (93–97%) of participants in the tirzepatide groups achieved HbA1c levels lower than 7.0% (53 mmol/mol), compared with 34% of the placebo group, and 26–62% versus 3% achieved HbA1c with the nondiabetic range, below 5.7% (39 mmol/mol)”
Significantly lowered glucose levels, improved insulin sensitivity, reduced weight, and a more favorable lipid profile… it keeps happening over and over again in humans, and in our vulnerable populations.
Folks, clinical evidence doesn’t get much better than this!
The BEST Tirzepatide Dose For Even Faster Weight Loss
Unlike other peptides where the optimal dose is based on the experience of 1-2 physicians and the collected forum testimonials of anonymous bioahckers, Tirzepatide’s dosing regimen is set in stone.
Here is the universal recommendation for the Tirzepatide dosage doctors use on their patients:
“Initial dose: 2.5 mg subcutaneously once a week
After 4 weeks: The dosage should be increased to 5 mg subcutaneously once a week.
If additional glycemic control is needed: The dosage should be increased in 2.5 mg increments after at least 4 weeks on the current dose.
Maximum dose: 15 mg subcutaneously once a week”
This regimen would make sense for someone who is fat and diabetic.
However, I would argue that going above 5 mg is overkill for a healthy individual as you would probably stop eating altogether.
From my experience, 2.5 mg of Tirzepatide a week was enough to mimic my higher doses of Semaglutide and I really didn’t need much else.
But don’t take it from me — take it from Dr. Eberwein, who has extensive experience with this medication since the second it was FDA-approved for treating Type 2 Diabetes:
“We are not 100% certain of the dose response and how each person responds to it.
HOWEVER, people already see results within the first month of using 2.5mg Tirzepatide per week (and sometimes within the first week itself).
100% of my patients have already switched over to Tirzepatide from Semaglutide, if they haven’t already.
The second thing you have to remember is the dosing escalation heavily favors Tirzepatide.
After the first dose escalation from 2.5 mg per week to 5 mg per week, you have already reached the minimum therapeutic dose.
The same can’t be said for Semaglutide, which needs two dose escalations to get to its minimum therapeutic dose of 1 mg weekly and at least two more dose escalations (for a minimum of four) to get to the highest dose of 2.4 mg weekly, which will take you 4-6 months.
That’s why many people note Semaglutide takes a much longer time for its effects to kick in.”
Try it and see for yourself, but I would be pleasantly shocked if you needed anything more than 5 mg of Tirzepatide weekly unless you are chronically FAT.
Tirzepatide’s Side Effects & Safety Profile

The side effects of Tirzepatide are well-known and not surprising as they are commonly observed with GLP-1 receptor agonists such as Semaglutide:
- Nausea
- Consipation
- Diarrhea
- Feeling full quickly
Outside of this, I wouldn’t be too concerned.
Dr. Eberwein has confirmed to me that fewer side effects are consistently seen with Tirzepatide in comparison to Semaglutide, and that’s in addition to the lower discontinuation rate.
It’s entirely plausible the GIP receptor agonist activity is what allows for fewer side effects yet faster weight loss at the same time.
And several meta-analysis reviews (here, here, here, here and here) found Tirzepatide to be safe for use in comparison to placebo, with no elevated risk of hypoglycemia yet slightly higher rates of discontinuation and diarrhea at the 10-15 mg dose range.
But with all of this in mind, you may have a couple of questions… which is why Dr. Eberwein has generously agreed to answer them below
Commonly Asked Questions About Tirzepatide
Q: Will Tirzepatide make my blood sugar too low?
A: No. Tirzepatide is almost like a glucose-independent way of modulating insulin.
Official directions make it clear that your habits are what will lower them…
“This medicine does not cause hypoglycemia (low blood sugar). However, low blood sugar can occur when you use tirzepatide with other medicines, including insulin or sulfonylureas, that can lower blood sugar. Low blood sugar also can occur if you delay or miss a meal or snack, exercise more than usual, drink alcohol, or cannot eat because of nausea or vomiting” (Source).
Q: Can I take this if I am not diabetic? The FDA doesn’t have Tirzepatide approved for use in non-diabetic people who are overweight/obese.
A: Absolutely!
While Tirzepatide is FDA-approved for treating diabetes right now, remember that it is on Fast Track Designation.
Therefore, it will likely get full approval in 6 months for weight loss in overweight/obese non-diabetic patients as we already have solid evidence of tackling the problem with GLP-1 receptor agonists (REMEMBER: Semaglutide is already FDA-approved for non-diabetic overweight people).
But since Tirzepatide has FDA approval for SOMETHING, physicians are allowed to use them off-label and have the full liberty to prescribe them for patients under two conditions:
(1) The patient has to be fully aware they are using a prescription medication for off-label purposes, and (2) the doctor has to use their best clinical judgment and explain their decision.
As Tirzepatide gets more popular, I suspect it will be overwhemingly used in non-diabetic people who have some kind of metabolic syndrome or need to get rid of the stubborn 10-20 pounds of fat they’re carrying around.
But even if you are not severely overweight, you can’t go wrong with improving your metabolic profile!
Q: What about the possible side effect of pancreatitis?
A: If you already have pancreatitis, you cannot take Tirzepatide and you should stop using it if you notice it developing (and seek immediate medical attention)
Although it’s something to watch out for as a possible contraindication, this was more prevalent with the very first GLP-1 receptor agonists to come out in the 2000s.
In my clinical experience, I have rarely seen this come up with either Tirzepatide or Semaglutide.
Q: Will Tirzepatide increase my chances of contracting thyroid cancer?
A: This scare came up when people started reading the official product monograph and noticed the following warning…
“Tirzepatide causes thyroid C-cell tumors in rats. It is unknown whether MOUNJARO causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as the human relevance of tirzepatide-induced rodent thyroid C-cell tumors has not been determined.
MOUNJARO is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)” (Source)
In my 30 years of practice I have yet to encounter a single person with MEN 2 or MTC.
If you have this, you will already know well in advance.
Furthermore, for those of you who don’t have any family history of thyroid cancer, please remember that rat studies do not always translate cleanly to human outcomes.
You would need to do a dose-dependency study for cancer effects, which has likely yet to be done.
Long story short – there is no need whatsoever for you to worry. 🙂
Q: Any 3rd generation weight loss drugs to look out for, or is this as good as it gets?
A: You have to remember that Semaglutide, one of the latest 1st-generation GLP-1 receptor agonists, JUST got FDA-approved for non-diabetic weight loss in 2021.
We now have Tirzepatide, arguably our 2nd-generation GLP-1 receptor agonist, on its way towards approval for the same condition in early 2023.
I’m incredibly excited as a physician because we are opening up a new era of no longer relying on stimulants, and now we are discovering how the gut hormones have such a significant effect on weight loss.
Even more amazing is how the weight loss we’re seeing from Tirzepatide in 1-2 months isn’t just from a lower calorie intake alone.
I’m convinced something else is happening to our metabolism that we don’t 100% understand yet.
Either way… if drugs can be THIS GOOD right now, the next 1-2 years of improvements on Tirzepatide (or possibly a new drug) is going to really blow us away.
As it stands right now, Tirzepatide is projected to become a $100 billion drug by 2035 and possibly tackle other conditions such as “kidney disease, sleep apnea, NASH, pre-diabetes and have cardiovascular uses” (Source).
Q: Anything you would recommend combining Tirzepatide with?
I haven’t personally tried this (I know Jay is in the middle of this experiment), but theoretically I could see Tesofensine helping burn more fat.
With my patients, I like to put them on an intermittent fasting program while on Tirzepatide because the reduced hunger makes it much easier for them to adhere to the program.
In my clinical practice I am to eventually wean patients off Tirzepatide so they can learn how to fast and control their hunger without relying on drugs.
I may give them extra B12 in rare cases to help with nausea, but this is just my clinical judgment and not really a recommendation wholly based in science.
Q: Is there such as thing as too-fast weight loss that’s unhealthy? What if a new version of Tirzepatide gives us 36% weight loss instead of 24% weight loss within the same time period?
A: I would not worry about this at all.
The human body will let you lose whatever you lose, and the first 5-10% will go real quick as some of it is water weight.
After that, the body’s metabolism will adjust into a state of homeostasis and the speed of weight loss will gradually slow down.
Because your body always “wants” to be in equilibrium and will find a way to maintain it, none of the medications you take for weight loss will ever lead to anything unhealthy.
In my clinical experience with hundreds of weight loss patients, there is no need to eat a diet extremely low in calories (and therefore low in protein) when you’re on Tirzepatide. You’re just going to eat less on a normal caloric deficit and that’s it.
Frankly, I don’t know how we’re getting such significant weight loss without slashing calories way too much.
But if you just keep protein high and your caloric intake at an appropriate deficit relative to your maintenance requirements while using Tirzepatide, you’ll be surprised at how fast and healthy fat loss can be.
Semaglutide or Tirzepatide – Which One Should I Take?
Here’s how I would go about it…
First, there’s absolutely no reason to combine them as they target the same receptor and you’re just engaging in overkill with no clinical benefit.
If you want to transition from Semaglutide to Tirzepatide… take a week-long break from Semaglutide, then start Tirzepatide at the lowest dose and then gradually increase it per my dosing instructions from earlier in the article.
Second, if you can get coverage from insurance, take whichever one you can get the coverage from.
Some people may have to choose between one or the other to save money, so don’t beat yourself up too much if Semaglutide is the only option in your budget right now.
Third, if money is not an issue and you are happy to pay out of pocket, Tirzepatide is clearly the superior choice.
The price is the around the same as Semaglutide, you still take it once a week, but the fat loss results are so much better.
CONCLUSION: Tirzepatide May Have Changed Weight Loss Forever

If Semaglutide was the weight loss breakthrough, then Tirzepatide is the breakthrough OF the original breakthrough!
Myself and Dr. Eberwein are seeing an insane amount of weight AND fat loss in a short time period — the inches lost and the mirror selfies do not lie.
And for someone like Dr. Eberwein, who has spent 17 years in the trenches of the weight loss industry, Tirzepatide’s novel mechanism of action has him elated for what the 3rd generation of weight loss drugs will bring.
America is overweight not because of a lack of information or direction, but because food is extremely addictive and tastes good.
Add in chronically elevated levels of stress, and it feels as if we can’t find ways to control our rising appetites and cravings.
But if we can give people a life-changing tool that puts the control back in their hands, it is 100x easier to adhere to any weight loss program.
This leads me to the tool I want to give to you right now…






